|
|
|
|
November 15, 2024 -Friday |
|
|
|
 |
|
|
|
|
NEURALSTEM REPORTS YEAR END 2017 FISCAL RESULTS AND BUSINESS UPDATE
Tuesday 03/04/2018
- Neuralstem Releases Full Data Set for NSI-189 MDD Trial Showing Patient Rated Outcome and Cognitive Benefits
- Nature Medicine Paper Demonstrates Benefit of NSI-566 on Paralysis in Non-Human Primates
GERMANTOWN, Md., April 3 (Bernama-GLOBE NEWSWIRE) -- Neuralstem, Inc. (Nasdaq:CUR), a biopharmaceutical company focused on the development of nervous system therapies based on its neural stem cell technology, reported its financial results for the year ended December 31, 2017.
“We remain committed to the development of NSI-566, our lead stem cell therapy candidate, and NSI-189 for major depressive disorder (MDD), which we believe offer novel modalities of treatments for patients that are not effectively treated by current therapies,” said Rich Daly, President & CEO. “We are preparing for the initiation of a clinical trial for NSI-566 in chronic stroke in China and have targeted mid-2018 to begin dosing. The recent findings of NSI-566 that were published in Nature Medicine were very encouraging, demonstrating NSI-566 led to a measurable improvement in forelimb function in injured animals. We look forward to continuing to explore its utility as a potential treatment for paralysis associated with spinal cord injuries.”
“We plan to formulate the clinical development path for NSI-189 in MDD after our meeting with the U.S. Food and Drug Administration which we expect to occur in the second half of 2018. NSI-189 may offer cognitive benefits in addition to antidepressant effects, which would distinguish it from other approved treatments,” Mr. Daly continued.
Clinical Highlights for Lead Clinical Programs
NSI-566, is a spinal cord-derived neural stem cell line that is being evaluated to treat paralysis associated with stroke, Amyotrophic Lateral Sclerosis (ALS) and chronic spinal cord injury (cSCI).
- The publication of a manuscript in Nature Medicine demonstrated that NSI-566 provided meaningful improvement in forelimb function in a non-human primate model following acute spinal cord injury. The study was performed at the California National Primate Research Center at University of California, Davis by Mark H. Tuszynski, MD, PhD, Professor of Neurosciences, Director of the Center for Neural Repair, an Attending Neurologist at the University of California, San Diego (UCSD). The full manuscript can be found here.
- In March, the Journal of Neurotrauma published preclinical data on NSI-566 spinal cord-derived neural stem cells in a study entitled, “Amelioration of penetrating ballistic-like brain injury induced cognitive deficits after neuronal differentiation of transplanted human neural stem cells.” These data showed robust engraftment and long-term survival of NSI-566 post transplantation in a rat model of penetrating ballistic-like brain injury. These are the first data from the 4-year proof-of-concept research program, funded by the United States Department of Defense, for NSI-566 in traumatic brain injury. The study was led by Ross Bullock, M.D., Ph.D., The Miami Project to Cure Paralysis, University of Miami School of Medicine.
NSI-189, a benzylpiperazine-aminopyridine, in clinical development for MDD and in preclinical development for Angelman syndrome, irradiation-induced cognitive impairment, Type 1 and Type 2 diabetes, and stroke.- At the 56th American College of Neuropsychopharmacology Annual Meeting last December, additional safety, efficacy and tolerability data from an exploratory Phase 2 clinical trial examining the efficacy of NSI-189 at 40 mg once daily (QD) and 40 mg twice daily (BID) compared to placebo for the treatment of MDD were presented in a poster entitled, “A Phase 2, Double-Blind, Placebo-Controlled Study of NSI-189 Phosphate, a Neurogenic Compound, Among Out-Patients with Major Depressive Disorder.” These additional results suggest that NSI-189 has antidepressant effects with cognitive benefits shown on both objective and subjective measures.
- Last August, Neuralstem was awarded approximately a $1 million Phase 2 Small Business Innovation Research grant by the National Institutes of Health (NIH) to conduct preclinical research into the potential of NSI-189 for the prevention and treatment of diabetic neuropathy.
- As previously announced in July 2017, NSI-189 failed to achieve statistical significance on its physician measured primary endpoint in an exploratory Phase 2 clinical trial examining the efficacy of NSI-189 at 40 mg once daily and 40 mg twice daily compared to placebo for the treatment of major depressive disorder. The study, which utilized the two-staged sequential parallel comparison design, did not meet its primary efficacy endpoint of a statistically significant reduction in depression symptoms on the Montgomery-Asberg Depression Rating Scale (MADRS). However, as reported in our topline results, the 40 mg QD dose was directionally positive on the MADRS and met statistical significance on several key secondary patient reported efficacy endpoints.
- Neuralstem intends to meet with the U.S. Food and Drug Administration to discuss the clinical development path for NSI-189 in the second half of 2018.
Corporate Highlights- In November, the Company appointed David Recker, MD, as Chief Medical Officer. Dr. Recker has more than 20 years of experience in drug development in multiple therapeutic areas including Central Nervous System disorders and cell therapy and has been involved in numerous aspects of clinical strategy development, including product registration and marketing support, clinical trial development and execution, data interpretation, key opinion leader development and support.
- In September, Cristina Csimma, Pharm.D, MHP, joined the board of directors. Ms. Csimma brings extensive senior leadership experience in the biopharmaceutical industry, including expertise in drug development and regulatory and commercial processes. In December, Xi Chen, Ph.D., was appointed to the Company’s Board of Directors by Tianjin, Neuralstem’s largest shareholder. Dr. Chen replaces Zhang Zhuo as the director appointee of the Series A Convertible Preferred Stock.
- Also in September, the Company was awarded two additional patents by the United States Patent and Trademark Office. These patents broadly protect methods for using neural stem cells to treat neurodegenerative disorders, a key component of the Company’s platform. The first new patent, U.S. Patent No. 9,744,194, covers methods of treating neurodegenerative disorders through transplantation of neural stem cells. The second new patent, U.S. Patent No. 9,750,769, covers neural stem cells engineered to express IGF-1, a neurotrophic molecule with broad therapeutic potential in the treatment of neurodegenerative disorders.
- In June, Neuralstem was added to the Russell Microcap® Index as part of the FTSE’s annual reconstitution of its family of U.S. indexes.
Financial Results for the Year Ended December 31, 2017
Cash Position and Liquidity: At December 31, 2017, cash and investments was $11.7 million as compared to $20.2 million at December 31, 2016. The $8.5 million decrease is due to cash used in operations and $3.8 million to pay down our long-term debt partially offset by $5.4 million of proceeds from the August 2017 public offering of common stock and warrants and $3.2 million of proceeds from the exercise of outstanding warrants.
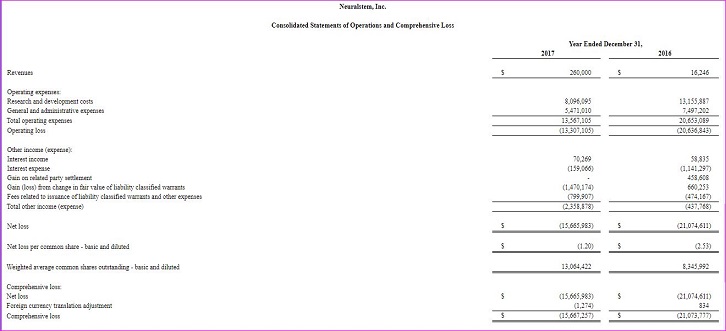
Operating Loss: Operating loss for the year ended December 31, 2017 was $13.3 million compared to a loss of $20.6 million for the same period of 2016. The decrease in operating loss for the year was primarily due to ongoing corporate restructuring and cost reduction efforts partially offset by increases in clinical trial expenses as the Company completed the Phase 2 clinical trial of NSI-189.
Net Loss: Net loss for the year ended December 31, 2017 was $15.7 million, or $1.20 per share (basic), compared to a loss of $21.1 million, or $2.53 per share (basic), on a split adjusted basis for the same period of 2016. The decrease in net loss was primarily due to a decrease in operating expenses and interest expense due to the maturity of long-term debt in April 2017 partially offset by non-cash expense related to the change in the fair value of our liability classified warrants.
R&D Expenses: The $8.1 million of research and development expenses for 2017 represents a $5.1 million, or 38% decrease over 2016 expenses. This decrease was primarily attributable to a $2.3 million decrease in personnel, facility and other expenses related to ongoing corporate restructuring and cost reduction efforts, a $2.1 million decrease in costs related to our completed NS-189 Phase 2 clinical trial and a $0.7 million decrease in non-cash stock-based compensation expense.
G&A Expenses: The $5.5 million of general and administrative expenses for 2017 represents a $2.0 million, or 27% decrease over 2016 expenses. This decrease was primarily attributable to a $1.2 million decrease in payroll and related expenses due to our ongoing corporate restructuring and cost reduction efforts and a $1.0 decrease in non-cash stock-based compensation expense partially coupled.
Liquidity: The Company expects its existing cash, cash equivalents and short-term investments to fund its operations based on our current operating plans, into the second quarter of 2019.
Neuralstem, Inc.
Consolidated Balance Sheets

About Neuralstem
Neuralstem is a clinical-stage biopharmaceutical company developing novel treatments for nervous system diseases of high unmet medical need. The Company has two lead development candidates:- NSI-189, is a small molecule in clinical development for major depressive disorder and in preclinical development for Angelman syndrome, irradiation-induced cognitive impairment, Type 1 and Type 2 diabetes, and stroke.
- NSI-566 is a stem cell therapy being tested for treatment of paralysis in stroke, Amyotrophic Lateral Sclerosis (ALS) and chronic spinal cord injury (cSCI).
Neuralstem’s diversified portfolio of product candidates is based on its proprietary neural stem cell technology.
Cautionary Statement Regarding Forward Looking Information
This news release contains “forward-looking statements” made pursuant to the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements relate to future, not past, events and may often be identified by words such as “expect,” “anticipate,” “intend,” “plan,” “believe,” “seek” or “will.” Forward-looking statements by their nature address matters that are, to different degrees, uncertain. Specific risks and uncertainties that could cause our actual results to differ materially from those expressed in our forward-looking statements include risks inherent in the development and commercialization of potential products, uncertainty of clinical trial results or regulatory approvals or clearances, need for future capital, dependence upon collaborators and maintenance of our intellectual property rights. Actual results may differ materially from the results anticipated in these forward-looking statements. Additional information on potential factors that could affect our results and other risks and uncertainties are detailed from time to time in Neuralstem’s periodic reports, including the Annual Report on Form 10-K for the year ended December 31, 2017 filed with the Securities and Exchange Commission (SEC), and in other reports filed with the SEC. We do not assume any obligation to update any forward-looking statements.
Contact:
Kimberly Minarovich
Argot Partners (Investor Relations)
212-600-1902
neuralstem@argotpartners.com
SOURCE : Neuralstem, Inc.
--BERNAMA
|
|
|
|
|
|