|
|
|
|
 |
|
|
|
|
NEURALSTEM REPORTS THIRD QUARTER 2018 FINANCIAL RESULTS AND PROVIDES BUSINESS UPDATE
Thursday 15/11/2018
- NSI-189 Received Orphan Designation for Treatment of Angelman Syndrome -
- Initiated Phase 2 Clinical Trial of NSI-566 for Treatment of Chronic Stroke -
- Second-Generation Neural Stem Cell Program NSI-532 Showed Positive Preclinical Results for Alzheimer’s Disease -
- Closed $2.1 Million Registered Direct Offering -
GERMANTOWN, Md., Nov 15 (Bernama-GLOBE NEWSWIRE) -- Neuralstem, Inc. (Nasdaq: CUR), a biopharmaceutical company focused on the development of nervous system therapies based on its neural stem cell and small molecule technologies, today provided a business update and reported its financial results for the third quarter ended September 30, 2018.
“During the third quarter of 2018 we have made progress with our programs, have taken steps to strengthen the balance sheet, and, perhaps most importantly, have determined how we will focus our efforts moving forward,” said Jim Scully, Interim Chief Executive Officer of Neuralstem. “We believe that proceeds from our recent offering, combined with our efforts to reduce cash burn, will allow us to pursue key development initiatives that will be detailed in the coming weeks.”
Clinical Highlights - In August, NSI-189 received from FDA the orphan designation for treatment of Angelman Syndrome. Angelman Syndrome (AS) is a rare congenital genetic disorder caused by a lack of function in the UBE3A gene on the maternal 15th chromosome. It affects approximately one in 15,000 people - about 500,000 individuals globally. Symptoms of AS include developmental delay, lack of speech, seizures, and walking and balance disorders. Patients with AS may never walk or speak and require life-long care. Life expectancy is normal which places a significant burden on patients and caregivers. There are currently no FDA-approved therapies for the treatment of Angelman syndrome.
- In July, the company announced initiation of a Phase 2 clinical trial to further evaluate NSI-566 in ischemic stroke. This follows the positive results of the open-label Phase 1 stroke study disclosed in a 2018 ISSCR (International Society for Stem Cell Research) abstract on June 23, 2018. The Phase 2 study is a randomized, double-blind, sham-surgery controlled study. It is intended to further test the safety and efficacy of NSI-566 to reverse paralysis in stroke patients with half of their body partially paralyzed. The trial is taking place at BaYi Brain Hospital in Beijing, China, and commenced on August 1, 2018.
- In October, the company announced publication of a manuscript in Scientific Reports showing that transplantation of NSI-532.IGF1 mitigates disease pathology and improves cognition in a mouse model of Alzheimer’s Disease. The study was performed at the University of Michigan by a team led by Dr. Eva Feldman, Director of the Program for Neurology Research and Discovery, and Research Director of the University of Michigan ALS Center of Excellence. NSI-532.IGF1 is the first candidate from the NSI-532 program, a second-generation cell therapy program which combines neural stem cells with neuroprotective proteins.
Corporate Highlights - In October, the company completed a registered direct offering of its securities which resulted in gross proceeds to Neuralstem of $2.1 million. Neuralstem intends to use the proceeds from this offering to further its clinical and preclinical programs, and for general working capital.
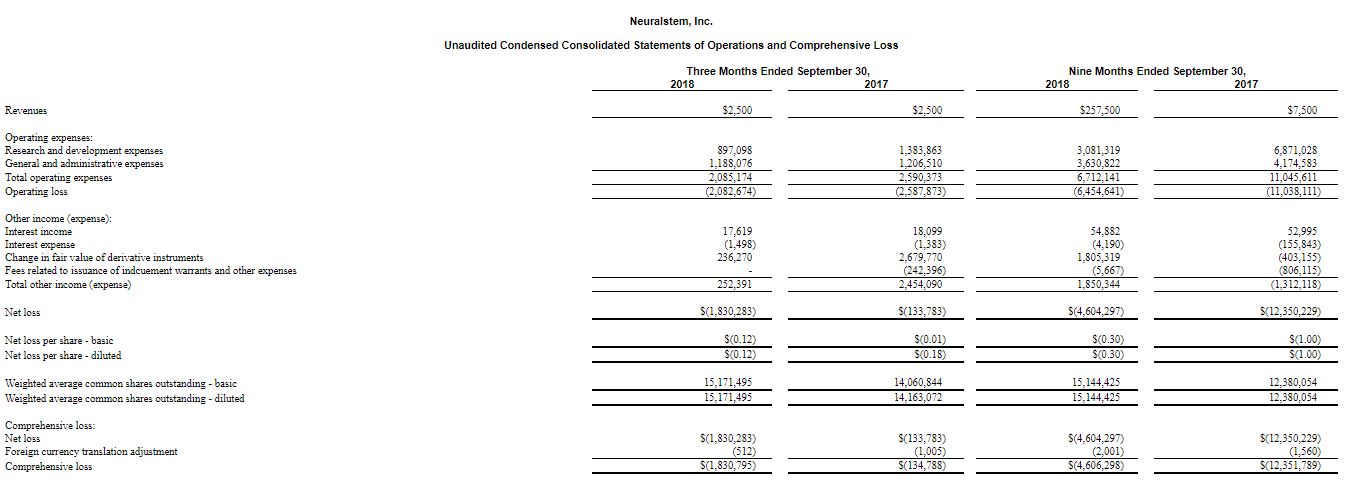
Financial Results for the Quarter Ended September 30, 2018 - Cash Position and Liquidity: At September 30, 2018, cash and investments was $5.7 million as compared to $7.1 million at June 30, 2018. The $1.4 million decrease reflects a loss for the period, which was somewhat ameliorated by management’s cost reduction efforts. The company expects its existing cash, cash equivalents and short-term investments to fund its operations, based on its current operating plans, into the second quarter of 2019.
- Operating Loss: Operating loss for the third quarter ended September 30, 2018 was $2.1 million compared to a loss of $2.6 million for the comparable period of 2017. The reduction in loss is attributed to reduced clinical development activity and management efforts to reduce overall expenses.
- Net Loss: Net loss for the third quarter ended September 30, 2018 was $1.8 million, or $0.12 per share (basic), compared to a loss of $0.1 million, or $0.01 per share (basic), for the comparable period of 2017. The increase in net loss was primarily due to the non-cash gains related to the change in the fair value of our liability classified warrants in 2017, partially offset by a decrease in our operating loss.
- Research and Development Expenses: The $0.9 million of research and development expenses for the quarter ended September 30, 2018 represents a 35% decrease over the comparable period of 2017. This decrease was primarily attributable to a decrease in personnel and facility expenses, and a decrease in clinical trial and related costs due to the completion of our NSI-189 Phase 2 clinical trial.
- General and Administrative Expenses: The $1.2 million of general and administrative expenses for the third quarter ended September 30, 2018 represents a 2% decrease over the comparable period of 2017. This decrease was primarily attributable to lower payroll and related expenses due to corporate restructuring and cost reduction efforts coupled with a decrease in non-cash share-based compensation expense which was offset by an increase in tax and insurance expenses and consulting and professional fees.
 Cautionary Statement Regarding Forward Looking Information Cautionary Statement Regarding Forward Looking InformationThis news release contains “forward-looking statements” made pursuant to the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements relate to future, not past, events and may often be identified by words such as “expect,” “anticipate,” “intend,” “plan,” “believe,” “seek” or “will.” Forward-looking statements by their nature address matters that are, to different degrees, uncertain. Specific risks and uncertainties that could cause our actual results to differ materially from those expressed in our forward-looking statements include risks inherent in the development and commercialization of potential products, uncertainty of clinical trial results or regulatory approvals or clearances, need for future capital, dependence upon collaborators and maintenance of our intellectual property rights. Actual results may differ materially from the results anticipated in these forward-looking statements. Additional information on potential factors that could affect our results and other risks and uncertainties are detailed from time to time in Neuralstem’s periodic reports, including its Annual Report on Form 10-K for the year ended December 31, 2017, and its Quarterly Report on Form 10-Q for the three, six and nine months ended March 31, June 30 and September 30, 2018, filed with the Securities and Exchange Commission (SEC), and in other reports filed with the SEC. We do not assume any obligation to update any forward-looking statements. Contact:Argot Partners (Investor Relations) 212-600-1902 neuralstem@argotpartners.com Source: Neuralstem, Inc.--BERNAMA |
|
|
|
|
|