KUALA LUMPUR, Aug 6 (Bernama) -- The National Pharmaceutical Regulatory Agency (NPRA), Ministry of Health Malaysia (MOH) would like to urge the public to refrain from buying and using the following cosmetic products which had been found to contain scheduled poison as below:
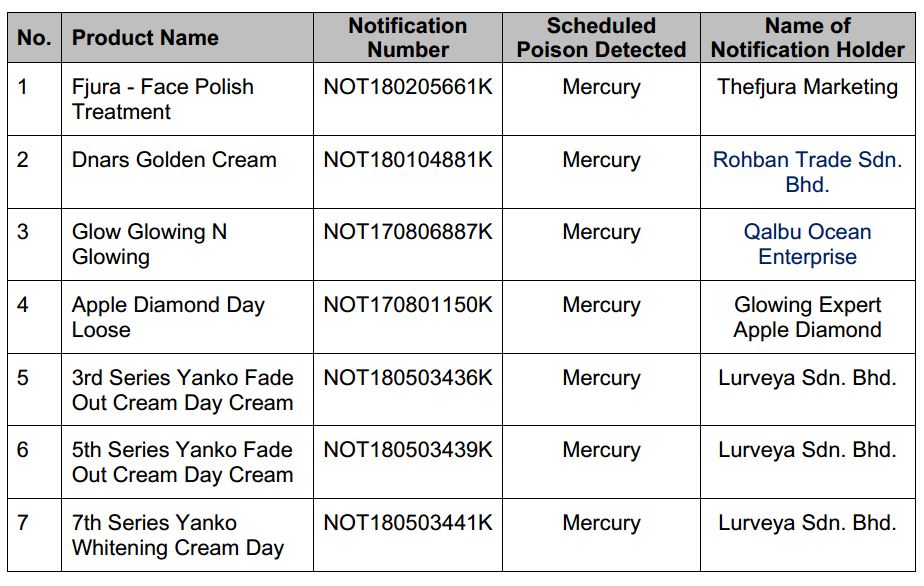
Notification of these cosmetic products have been cancelled from the market by the Senior Director of Pharmaceutical Services, MOH following the detection of scheduled poison. These cosmetic products are no longer allowed to be sold in Malaysia.
DESCRIPTION ABOUT SCHEDULED POISON Mercury is prohibited in cosmetic products due to its hazardous effects on human health. It is readily absorbed through the skin on topical application and tends to accumulate in the body. Exposure to mercury can cause skin rashes, memory loss and muscle weakness while high exposures may result in damage to the brain and kidneys. It is also extremely toxic to unborn children.
WARNING TO VENDORS AND DISTRIBUTORS OF THESE COSMETIC PRODUCTS All sellers and distributors must stop selling and distributing of these products immediately.
Selling or distributing these cosmetic products is an offence under the Control Of Drugs and Cosmetics Regulations 1984.
Any individual who commits an offence under these Regulations can be fined up to a maximum of RM25,000 or to imprisonment for a term not exceeding 3 years or both, and for a second or subsequent offence, shall be liable on conviction to a fine not exceeding RM50,000 or imprisonment for a term not exceeding 5 years or both. A company found guilty can be fined up to RM50,000 for the first offence and fined up to a maximum of RM100,000 for subsequent offences.
ADVISORY TO PUBLIC Consumers are advised to stop using these products and seek further advice from healthcare professionals, if experiencing any unpleasant effects or adverse events.
The public are encouraged to check the notification status of a cosmetic product via NPRA’s official website at
http://www.npra.gov.my/ Thank you.
DATUK DR. NOOR HISHAM ABDULLAH
DIRECTOR GENERAL OF HEALTH MALAYSIAPlease click here for
APPENDIX ASource: MINISTRY OF HEALTH MALAYSIA
FOR MORE INFORMATION, PLEASE CONTACT:
Name: BAHARUDIN BIN MOHAMAD
Pegawai Perhubungan Awam
Unit Komunikasi Korporat
Tel : 03-88834536 / 017-2635008
--BERNAMA